Vesicovaginal and Urethrovaginal Fistulas
Authors
INTRODUCTION
This chapter is dedicated to our friend and teacher, Dr Thomas Elkins.
The first description of the repair of vesicovaginal fistulas (VVFs) pre-dates Sims’ classic work in 1852, which reported a successful operation in a slave after several failed attempts.1 Since then, numerous techniques have been reported. The occurrence of VVF and urethrovaginal fistulas can be one of the most troublesome complications of obstetric trauma and pelvic surgery. Appropriate management regarding timing of repair and surgical approach remains controversial.
EPIDEMIOLOGY/ETIOLOGY
The majority of the literature reflects a particular center’s experience. Lee and colleagues found 82% of their institution’s fistulas to have resulted from gynecologic surgery, 8% from obstetric procedures, 6% from pelvic radiotherapy, and 4% from trauma.2, 3 Goodwin and Scardino found 74% of their cases to be of gynecologic origin, 14% of urologic origin, and 12% from radiation injury.4
The frequencies and causes of VVF also reflect the culture and geography. Kelly showed that in England, 95% of the VVFs occurred with nonobstetric causes.5 In Nigeria, 98% of the VVFs were secondary to obstructed labor.6 Success rates at repair are therefore difficult to compare, and a major contribution to the differences reported also probably reflects variations in the populations studied.
Predisposing risk factors for VVF include a history of pelvic irradiation, cesarean section, endometriosis, prior pelvic surgery or pelvic inflammatory disease, diabetes mellitus, concurrent infection, vasculopathies, and tobacco abuse. In developing countries, obstetric trauma remains the leading cause of VVFs. In some countries in Africa, it is customary for early marriages involving adolescent girls to be contracted prior to the commencement of their menses. In sub-Saharan Africa, nearly 50% of the women are married by age 18, some by age 15 or younger. A recent study from Katsina, Nigeria, found that primiparous girls who married during early adolescence were more likely to experience VVF than those who married at an older age. Women without formal education and those married to men with unskilled jobs were 14 times more likely to sustain a VVF than their cohorts.7 In this population, there can be grave social consequences of VVF, including divorce, poverty, and depression.
Obstructed labor is a common complication of childbirth and may lead to obstetric vesicovaginal fistula which are uniquely human. Wittman and Wall have reviewed the changes imposed on the pelvis by assumption of an upright posture and bipedal locomotion.8 Vesicovaginal fistula occurs when the baby’s head is unable to pass through the bony pelvis and impacts against an edematous distended anterior vaginal wall with resulting pressure necrosis. Introital stenosis secondary to female circumcision, cephalopelvic disproportion (from reduced pelvic dimensions of early childbearing), an android pelvis, malnutrition, orthopedic disorders including rickets, and hydrocephalus contribute to dystocia. Fistulas may be caused by forceps, destructive instruments used to deliver stillborn infants, or surgical abortion. Symphysiotomy, the use of postpartum vaginal caustic agents, and self-inflicted “Gishiri cuts” also have a role.
In countries with modern obstetric care, VVF is most commonly associated with pelvic surgery. The majority of VVFs are related to procedures performed by obstetrician-gynecologists; particularly total abdominal hysterectomy. The remainder are divided among urologists and general, colorectal, and vascular surgeons.9 The overall incidence of urinary tract injuries during pelvic surgery is estimated to be 0.33%. The most common operation is abdominal hysterectomy and the most common indication is benign leiomyoma. Cystotomy and VVF account for more than three-quarters of the injuries. Predisposing factors for bladder injury are coexisting pelvic pathology, distortion of normal anatomy, previous pelvic surgeries, adhesions and extended surgery such as radical hysterectomy. Indeed, the incidence of bladder injury during radical hysterectomy is three times higher than with simple hysterectomy. However, the largest portion of VVF is associated with simple hysterectomy because of the shear volume of surgery for benign indications.10
The etiology of VVF at the time of hysterectomy is uncertain. Some are the result of an unrecognized bladder laceration at the time of dissecting the bladder off the cervix. Even cystotomies that are repaired have a risk of fistula formation. A study in dogs report by Sokol and colleagues showed that double layer closure of cystotomy was superior to single layer closure.11
A fistula may also arise from avascular necrosis secondary to crush injury or erosion of a vaginal cuff suture into the bladder. While vaginal cuff sutures that are placed through the bladder are postulated to produce fistula, Meeks and coworkers, in a rabbit model, demonstrated that suture material placed through the vaginal cuff and the bladder was not associated with the development of fistulas.12 A fistula may also follow an uncomplicated operation as the result of a pelvic hematoma that ruptures into the bladder postoperatively. Devascularizing the bladder or vaginal cuff could lead to fistula formation and can be minimized with mobilization of tissue planes. Tancer’s suggestions to avoid injury to the bladder during total abdominal hysterectomy include the use of a two-way indwelling catheter, sharp dissection to isolate the bladder, an extraperitoneal cystotomy when the dissection is difficult, retrograde filling of the bladder when injury is suspected, and repair of an overt bladder injury only after mobilization of the injured area.13 Filling the bladder can also help define the border of the bladder otherwise displaced by a prior surgery or a lower uterine segment fibroid.
Radiation-induced fistulas are commonly associated with treatment for carcinoma of the cervix or other pelvic malignancies.14 Fistulas may appear during the course of radiotherapy (usually from necrosis of the tumor itself) or after treatment is completed. Late fistulas arise secondary to endarteritis obliterans within the first 2 years. It is essential to rule out recurrent malignancy with biopsies.
There are case reports of VVFs caused by vaginal foreign bodies, direct trauma from masturbation or automobile accidents, bladder calculi, forgotten vaginal pessaries, endometriosis, and infections such as tuberculosis, schistosomiasis, syphilis, and lymphogranuloma venereum, and from idiopathic congenital causes.15, 16, 17, 18 Sexual trauma through coerced vaginal penetration and even consensual sexual intercourse have been reported to have led to VVF.19 Idiopathic congenital VVF is usually associated with other genitourinary anomalies.20 Placement of transobturator midurethral slings are touted as being less likely to cause bladder injury. However, recent reports have documented VVF following trauma to the bladder with trocar placement and with the presence of a foreign body in the bladder; the latter may be caused by directly placing the tape through the bladder or erosion of the material into the bladder wall.21, 22
Urethrovaginal fistulas may occur postpartum and are associated with operative vaginal delivery, after surgery for urethral diverticulum, anterior vaginal wall prolapse, or urinary incontinence, and after radiation therapy. Pressure necrosis resulting in a urethrovaginal fistula can occur with a prolonged indwelling transurethral catheter. Urethrovaginal fistulas may also be congenital.23
CLINICAL PRESENTATION
Kursh and associates examined the records of 12 patients who had VVF develop after total abdominal hysterectomy.24 Most of the patients had excessive postoperative abdominal pain, distention or paralytic ileus, or both. Hematuria and symptoms of irritability of the bladder were also noted in the fistula group, and prolonged postoperative fever and increased white blood cell count occurred more often. The clinical course observed in these patients with VVFs suggests that many of them had an unrecognized injury to the bladder resulting in urinary extravasation. Theoretically, with early recognition, it may be possible to avert the formation of a VVF. However, Tancer described a series of 110 posthysterectomy fistulas, 24 of which occurred despite intraoperative recognition of bladder damage and prompt repair.13 More than 21% of patients in this series formed a VVF despite preventive maneuvers and repair of intraoperative bladder injury.
The most common presenting feature of VVFs is continuous leakage of urine from the vagina. The size and location of the fistula determine the degree of leakage. Patients with small fistulas may void normal amounts of urine and notice only slight position-dependent drainage. Alternatively, they may have leakage only at maximal bladder capacity. The patient may experience recurrent cystitis or pyelonephritis; unexplained fever; hematuria; flank, vaginal, or suprapubic pain; and abnormal urinary stream. Those with larger fistulas may not void transurethrally and may have total incontinence. Urinary leakage may make the patient a social recluse, disrupt sexual relations, and lead to depression, low self-esteem, and insomnia.
The leakage of urine may cause irritation of the vagina and vulvar mucosa, and perineum and usually produces a foul ammonia odor. Phosphate encrustations may be noted in more neglected cases. These crystals serve to further irritate what can be already compromised tissue.
ASSESSMENT
In many patients the diagnosis is obvious. A complete urologic investigation is mandatory though, especially to rule out ureterovaginal fistula. In one series, 12% of patients with VVFs had associated ureterovaginal fistulas.4 The investigation should include a speculum examination with collection of vaginal fluid for urea concentration. A urinalysis and urine culture will permit the physician to treat an infection. Intravenous urography may aid in localizing the fistula and determining the adequacy of renal function. Urethrovaginal fistulas are usually easily distinguished on examination. Otherwise, the use of a Tratner catheter and contrast medium may aid in the diagnosis of a urethrovaginal fistula. A caveat: Women with urethrovaginal fistulas may frequently have a fistulous communication between the bladder and the vagina as well. Lee and colleagues found that 10 of 53 patients (19%) with a urethrovaginal fistula had a separate VVF.3
All patients should undergo cystourethroscopy. The exact location (in relation to the ureteral orifices), size, and underlying cause of the fistula need to be determined. Additional fistulous communications need to be ruled out to reduce the opportunity for surgical failure. The bladder neck should be examined thoroughly and any associated loss of urethral tissue should be noted. Liquid-based cystoscopy may be impossible with larger fistulas. The vagina can be packed with gauze or, with the patient in jackknife position, the bladder can be allowed to fill with air and dry cystoscopy may be performed.
Various dye tests can be performed to elucidate the presence of a urogenital fistula.25 Authors have described a double dye test utilizing 1% carmine red solution instilled into the bladder and indigo carmine injected intravenously. Moir described a three-tampon test that may aid in localizing the fistulous tract.26 The vagina is packed with three tampons at different levels in the vaginal vault. Findings can be misleading. Excretion of indigo carmine depends on intact renal function, and occasionally red dye can reflux up a ureter and gain entry into the vagina via a ureterovaginal fistula, a false-positive result. Alternatively, oral phenazopyridine may be used to stain the urine orange, a tampon can be placed into the vagina, and the bladder can be catheterized, emptied, and filled with methylene blue/saline solution. After 10 minutes, the bladder is emptied and the tampon removed. An orange stain at the top of the tampon indicates a ureterovaginal fistula, and a blue stain is consistent with a VVF. Instillation of sterile milk into the bladder is recommended by some because it may be more easily seen. If a fistula cannot be documented by placing dye into the bladder, it is possible to instill methylene blue into the vagina and document that the urine is stained blue. Dyes are an excellent way of documenting a fistula in the office setting, but further testing is rarely avoided because of the need to define the fistula completely.27
The use of computed tomography (CT) with intravaginal contrast media to detect a VVF has been reported with limited success.28 CT scanning may be most beneficial in discerning the etiology and the extent of existing disease prior to surgical correction.
Color Doppler ultrasound with contrast media has been described as a diagnostic tool for VVF.29 Sonography was positive in 11 of 12 (92%) patients with VVF. The benefits are that is it easy to learn, is noninvasive, requires no radiation exposure, and can evaluate the distance from the fistula to the ureteral orifices. Transvaginal sonography findings correlated well with intravenous urography, cystogram, cystosocopy, and surgical findings.30
Hilton argues for the use of urodynamics before surgical repair of urogenital fistulas to establish abnormal lower urinary tract function in such patients.31 Of the 38 patients evaluated, 47% had genuine stress incontinence, 40% showed detrusor instability, and 17% impaired bladder compliance. The overall incidence of functional abnormality was highest in the patients with urethral or bladder neck fistulas. After surgical treatment of the fistulas, most patients became continent and free from lower urinary tract symptoms. Those who had urethral or bladder neck fistulas had more residual detrusor instability. These findings are relevant to the counseling of patients before repair and may be of medicolegal importance.
ANATOMIC CONSIDERATIONS
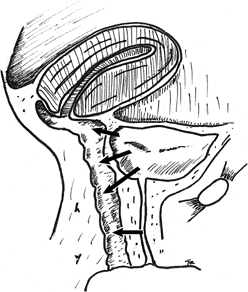
Posthysterectomy fistulas are usually supratrigonal, medial to both ureteral orifices, and lie within the vaginal vault at the vaginal cuff (Fig. 1). Fistulas from obstetric causes may be located more distally, typically are larger, and are more commonly associated with a urethral injury. Obstetric fistulas have been classified according to their anatomic location in relation to the cervix.
PREOPERATIVE CARE
Cystitis, vaginitis, and perineal dermatitis should be treated with the appropriate agent. First-line intervention is use of perineal pads to contain the uncontrollable and constant leak of urine. Instinctively, most women use menstrual hygiene products. These products are overwhelmed by the large volume of fluid often associated with urinary fistula. Women should be encouraged to use continence products which are designed to manage larger volumes. Local wound care is important, and frequent pad changes are required to minimize inflammation, edema, and vulvar irritation. Zinc oxide ointment is especially helpful in the treatment of perineal dermatitis. Keeping the perineum and vulva as dry as possible is comfortable for the patient. Inventive collection and drainage systems have been described for this purpose.32
Spontaneous closure of some vesicovaginal fistula with bladder drainage alone has been reported. Factors that may impact success are size, and interval from causative insult to initiation of drainage. Interval to drainage is postulated to correlate with epithelialization of the fistulous tract which interferes with healing.33 Denuding the tract with electrosurgery, laser or direct abrasion has been advocated as an adjunct to catheter drainage alone.34 The denuding of the tract has also resulted in closure of the defect. Estrogen therapy may also be used provided there are no contraindications. There is no convincing evidence that the use of corticosteroids improves the tissues and may interfere with healing when an early repair is attempted.35 Hyperbaric oxygen treatment has also been described as an adjuvant treatment in radiation-induced fistulas.36 These fistulas will not heal spontaneously or with conservative therapy, and surgical correction is required.37 Obviously, in the malnourished patient, her nutrition needs to be optimized and anemia needs to be corrected prior to surgery.
SURGICAL TECHNIQUE
Symmonds recommends several surgical principles to improve the success rate of any technique for VVF repair: wide mobilization of the bladder, excision of all scar tissue at the risk of increasing the size of the fistula in an attempt to create a “fresh bladder injury”, a tension-free layer closure of the bladder and the vagina, nontraumatizing technique, and good hemostasis with complete bladder drainage postoperatively.38 Most authors agree that the best chance at closure of the fistula is at the first attempt.
In general, the vaginal approach avoids the potential morbidity associated with abdominal surgery and is believed to provide a quicker recovery and a more cosmetic result. Lee and colleagues3 and Tancer13 report vaginal success rates of 98% and 100%, respectively. However, there are circumstances for which an abdominal approach has traditionally been favored: larger fistulas; fistulas located high on the posterior wall; fistulas adjacent to the ureters, where improved exposure is necessary; to correct concurrent intraabdominal pathology if it exists; and in cases in which the vaginal vault is narrow or deep which precludes adequate access to the fistula. Involvement of the ureter may require an abdominal approach to facilitate reimplantation or the placement of stents. Patients with a small or contracted bladder or a very large fistula may require augmentation with bowel. A combined vaginal and abdominal approach can be helpful in certain circumstances. The authors have described a combined approach to the repair of a large fistula secondary to a vaginal foreign body that involved the trigone.39
Vaginal repair
The patient is placed in lithotomy position. Choice of supporting stirrups is a matter of physician preference. The patient is examined under anesthesia. The ureteral orifices may be catheterized cystoscopically if there is concern about the ureters, especially if they lie at the edge of the fistula or if the fistula is high in the vaginal vault. Labial retraction sutures and an episiotomy or Schuchardt incision may be helpful for a small introitus and vaginal vault. Placement of stay or traction sutures at the margins of the fistula or the insertion and inflation of a Foley or Fogarty catheter helps to identify the fistula’s edges and may bring the tract closer to the surgeon. Some fistula surgeons infiltrate the vaginal mucosa with saline solution or a dilution of epinephrine (1:200,000) to aid dissection and decrease oozing.
There is no consensus on the need to excise the fistulous tract. Some authors advocate its total removal, whereas others prefer not to débride the margins, thereby avoiding an increase in the size of the defect. Iselin and colleagues advocate the excision of the fistula tract and vaginal cuff scar, enabling the surgeon to suture viable tissues in every layer to promote wound healing, obviating the need for interpositional flaps or grafts.40 They had a 100% cure rate on first attempt. Flynn reviewed patients who had excision of the vaginal cuff scar at the time of VVF repair. Patients had a very high degree of success without postoperative irritative voiding symptoms or dyspareunia.41
In his series of 65 transvaginal repairs, Raz did not excise the fistula tract in any patient and had no apparent adverse effects.42 Cruikshank did not excise the tract in his series of 11 patients and had a 100% cure rate.43 Zacharin warns that excision of the fistula scar markedly increases the risk of operative failure.44 Elkins and coworkers45 and Lawson46 also advised against excising the tract in large obstetric fistulas.
The two transvaginal techniques commonly performed are the flap-splitting technique and the Latzko procedure. The flap-splitting technique involves wide mobilization of the vaginal mucosa from the edge of the fistula. The bladder is closed in two layers. The first is submucosal with interrupted Lembert sutures. A second layer is used to close the muscularis and reduce tension on the first suture line. If the defect is in the trigonal area, the repair should be in a transverse direction, because a vertical closure may draw the ureters to the midline and lead to kinking or obstruction. The authors prefer to close the vagina using interrupted sutures to preserve vaginal length. The flap-splitting technique does not foreshorten the vagina and has a success rate equivalent to that of the Latzko procedure. Martius47 described the use of this technique for small fistulas and Raz48 reported a 100% cure rate in his series of 20 patients. Disadvantages include the possibility of mobilizing the tissues too much, leading to avascular necrosis of suture lines or incorporating the ureters into the closure.
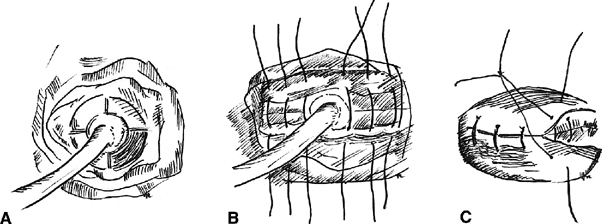
The Latzko partial colpocleisis is championed by many49, 50 (Fig. 2). An elliptical portion of vaginal mucosa is mobilized around the fistula tract, at least 2.5 cm in all directions. The pubovesical fascia and vaginal mucosa are closed in layers, using interrupted sutures. The vesical edges of the fistula are not denuded. The posterior vaginal wall becomes the posterior bladder wall and reepithelializes with urothelium. Because the bladder musculature is not sutured to itself, there is no tension across the suture lines. Success rates greater than 89% are reported for the first attempt. Other advantages include short operating time, minimal blood loss, and low postoperative morbidity. It is especially effective in patients with radiation-induced fistulas. Disadvantages include a loss of vaginal length and possible interference with sexual function.
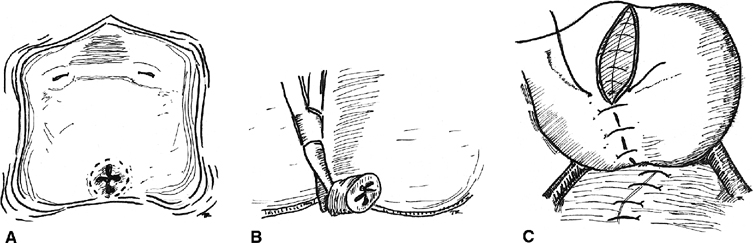
Interposition flaps or grafts may be used in larger or recurrent fistulas or those involving the urethra or bladder neck. Pedicle flaps bring additional blood supply, improve the lymphatic drainage, and distance suture lines. In 1928, Martius first described the use of the labial fat pad as an interposition graft.51 The vascular supply to the graft inferiorly is from the internal pudendal artery and superiorly is the external pudendal artery (Fig. 3). The key is the appreciation and preservation of one of these vascular bundles. Birkhoff and associates reported a 100% success rate in six patients with transvaginal repairs of VVF using the Martius technique.52 Elkins and colleagues reported a success/closure rate of 96% (24 of 25 procedures) in a series of mostly postobstetric injuries.53 Alternatively, a gracilis muscle flap may be used as first described by Ingelman-Sundberg,54 and later modified by Hamlin and Nicholson.55 The technique involves dissection of the gracilis muscle and separation of its attachment to the medial condyle of the femur. The blood supply to the muscle from the femoral artery is preserved, and the graft may be tunneled to the introitus and sutured into place at the fistula. Less invasive adjuvants to repair include placing a fold of peritoneum over the fistula site or interposition of dura mater or other biograft.56, 57
|
The repair should be watertight and tested with the instillation of methylene blue or indigo carmine into the bladder. The authors prefer to leave a pack in the vagina for 24 hours postoperatively and continuously drain the bladder with a 16 French Silastic suprapubic catheter for 3 weeks and discontinue the catheter after the patient successfully passes voiding trials. The patient is maintained on prophylactic antibiotics while the catheter is in situ.
In general, the repair of urethrovaginal fistulas is often more difficult than the closure of VVFs and may leave the patient incontinent. It is generally reported that the success rate of urethrovaginal fistula repair is 73–100%.58, 59 The same principles of VVF repair apply to the repair of urethrovaginal fistulas: wide mobilization of tissue planes, layered closure, and the use of interposition grafts when appropriate.
Noble originally described urethral reconstruction using bilateral vaginal flaps formed into a tube around a catheter.60 Skin grafted from the labia aided in maintaining cosmesis. Birkhoff and associates believe that the reestablishment of continence is facilitated by the use of the Martius interposition flap.52 In addition, Gray found that 50% of his patients were incontinent postoperatively without this added intervention.58 Moir described using suburethral buttressing sutures to aid with recovery of continence.61 Symmonds and Hill achieved continence in 37 of 50 patients (74%) with significant urethral destruction by constructing a neourethra, using smooth muscle that remains in the urethral “roof” and the creation of a Martius-type flap to aid with tension-free closure.62 They advocate delaying a retropubic suspension because concomitant suspension may lead to disruption or attenuation of the suburethral repair or devascularize the repair. There may also be difficulties associated in preselecting patients who would go on to require a subsequent suspension. Leach has described a simultaneous needle suspension for patients in whom stress incontinence is associated with a urethrovaginal fistula.63 A pedicle buttock flap has also been described for the repair of larger urethrovaginal fistulas.64 Fernandes and coworkers have reported the use of an anterior advancement flap of bladder and turning it into a tube to reconstruct an entire urethra.65 Patch grafts of bladder mucosa have also been used with satisfactory results for urethral reconstruction in a series by Omo-Dare.66
Abdominal repair
The patient should be in low lithotomy position with vaginal access. Incision type is also a matter of physician preference. The advantage to a low midline incision is that omentum can be mobilized for a graft, and it can be extended easily, although access to the omentum can be achieved with Maylard or Cherney incisions. Simple fistulas can be repaired transvesically (extraperitoneal), but an intraperitoneal approach is preferable for more complicated fistulas.
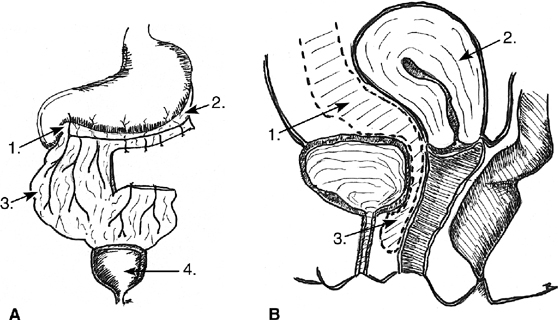
In the transvesical technique, the bladder is opened at the dome, the fistula is excised, and the bladder muscularis is mobilized off the vagina. The defects are then closed in layers. Exposure may be difficult, and because of this, many surgeons prefer the intraperitoneal approach. Much of the early work with this technique was pioneered by O’Conor and associates67, 68, 69 (Fig. 4). The bladder is bisected vertically down to the fistula tract. All scar is excised, and the bladder is widely mobilized off the vagina. The bladder and vagina are closed in layers. Whereas the O’Conor technique requires extensive dissection, Ostad and colleagues described an abdominal approach to repair with a free bladder mucosal graft in a series of six patients.70 There is reduced need for extensive dissection, and it is useful for repairs close to the ureters and in large or recurrent defects.
The use of vascularized tissue grafts can be helpful in ensuring a successful repair. One of the more popular graft sources is the omentum (Fig. 5). It has a dual blood supply, and some surgeons recommend dividing the splenic vessels on the left to help with mobilization. Others prefer to base the pedicle on the gastroduodenal vessels of the right side. Rectus muscle has also been used as graft for abdominal repairs. In a review, the use of interposition flaps was evaluated for VVFs of benign and malignant etiologies.71 All repairs with interposition grafts were successful without regard to etiology, whereas only 63% and 67% of repairs were successful for benign and malignant fistulas, respectively. Bladder mucosa can also be harvested from the dome of the bladder and placed over the repaired fistula. This adds a layer of tissue between the suture lines of the bladder and vagina.72 The authors recommended that transabdominal VVF repairs be performed with an interposition flap regardless of the appearance of healthy surrounding tissues and etiology.
Minimally invasive repair
While the majority of patients with VVF will have traditional surgical approaches, several nonsurgical therapies have shown promising results when used to close fistula smaller than 8 mm. Several minimally invasive approaches have been reported to be successful in closing VVFs smaller than 8 mm. Fibrin occlusion of a VVF was first reported by Pettersson and coworkers in 1979.73 To protect the fibrin plug, an indwelling urethral catheter was left in situ and the patient was give tranexamic acid to block fibrinolytic activity for 8 weeks. In a recent case report, Morita and Tokue describe a successful endoscopic closure of a 5-mm radiation-induced VVF with fibrin glue and bovine collagen.74 This required electrofulguration of the tract. Stovsky and associates retrospectively reviewed a series of 15 patients who were treated with electrocoagulation for VVF of a few millimeters or less.75 Fulguration was successful as the sole treatment modality in 9 of 12 patients (75%). Dogra and Nabi described using a cystoscopic approach and the neodynium:yttrium-aluminum-garnet (Nd-YAG) laser to fulgerate the fistula tract.76 Falk and Orkin reported on 10 patients treated by fulguration with a Bugbee electrode followed by 10 days of catheter drainage.77 All patients with fistulas of 3 mm or less in size were cured, whereas fulguration in two patients with 6-mm fistulas failed. The latest addition to the armamentarium of therapies is tissue bioglues. Cyanoacrylic glue and fibrin glue have been applied endoscopically, percutaneously and intravaginally. Success seems to be highest in fistulas which are relatively narrow and which have a longer tract.78, 79, 80 Aycinena reported use of an ordinary metal screw forced through the vagina and into the bladder as a means of denuding the epithelia of the fistula tract and promoting healing. Seven patients were successfully managed in this way.34
Minimally invasive surgical approaches have been used to close VVFs. Novel endoscopic suturing techniques led McKay to try transurethral suture repairs of VVFs.81 By transurethrally inserting a 5-mm laparoscopic sleeve alongside a 17 French cystourethroscope with a 30-degree lens and utilizing an extracorporeal knot tying technique, he was successful in two patients who had failed prior repair attempts. Continued success prompted him to report 5 years of experience in which he outlined selection criteria, instrumentation, a slightly modified technique and potential pitfalls.82
The O'Conor technique for abdominal repair has been accomplished with laparoscopy. Several authors have reported a high success rate using their individual variations of the O'Conor technique including interposition of an omental flap and rotational bladder flaps to prevent apposition of the vaginal and bladder suture lines.83, 84, 85, 86, 87
Comparison of techniques
Randomized clinical trials which compare the techniques of VVF repair have not been done. Available data suggest that success rates for each technique are comparable. In a review of the literature Angioli et al. found that the vaginal approach achieved comparable results to other approaches, while minimizing operative complications, hospital stay, blood loss, and postoperative pain. They therefore advocate the vaginal approach. Additionally, they feel it is acceptable to approach a second repair through the vaginal route even if the first vaginal approach resulted in failure.88 Dorairajan and colleagues compared their results from Latzko repairs and laparoscopic O'Conor repairs in a retrospective manner. The techniques were comparable in terms of morbidity, operative time, blood loss and patient pain. They recommended the vaginal approach over the more complex procedures such as the laparoscopic repair. They further recommended the vaginal approach over transurethral suture cystorrhaphy.89 Currently, the abdominal approach is favored only when the ureters are involved and may require re-implanation.90
Timing of repair
Optimal timing of repair remains controversial, Melah and colleagues reviewed this topic giving pros and cons of each technique.91 A longstanding tenet is to wait a minimum of 3 months after hysterectomy or the last attempt at repair, because inflammatory or necrotic fistula margins have been judged responsible for surgical failure. Others propose that early intervention is safe. Collins and coworkers, in a series of 38 patients all of whom had a transvaginal repair within 60 days after diagnosis, reported a 28% failure rate.92 Persky and associates have also been advocates for early repair, and they reported a series of seven patients who underwent successful fistula repair within 7–10 days of the antecedent surgery.93 Cruikshank also had success in 10 of 11 fistulas in his series repaired between 10 and 35 days after a hysterectomy.43 The average time to fistula repair in Iselin’s series was 16 weeks with 100% cure rate.40 Wein has attributed surgical failures to an inadequate delay until repair.9, 14 Lee and colleagues recommended delaying surgery to increase the success rate of the first attempt at repair.3 Angioli and colleagues have recommended waiting 4–6 weeks from the development of the fistula. Those associated with radiation should be delayed even longer.88
Most authors agree that treatment should be tailored to the individual patient and that optimum time for repair involves resolution of necrotic tissue and inflammatory changes and stabilization of the size of the fistula. Higher failure rates are associated with repairs on tissue that is acutely inflamed, edematous, ischemic, or necrotic. Scar excision may allow earlier repair because the inflamed cuff margins may be cut back to healthy tissue. Some fistulous communications recognized immediately postoperatively or after trauma with minimal inflammatory changes may lend themselves to early repair. In irradiated tissues, the interval to repair should be longer.
If the fistula is recognized within the first 48 hours postoperatively, the tissue should be more mobile, have less inflammation, and be amenable to early repair. Fistulas arising several days after surgery are frequently accompanied by significant edema, inflammation, and induration, making early repair less successful. An interval of 3 months from injury to repair in obstetric and surgical fistulas will allow the inflammatory reaction to subside before proceeding with repair. Up to 1 year may be required for improvement in the tissues in radiation-induced fistulas prior to repair. Prolonged catheterization may also lead to spontaneous closure in small fistulas, but the outcome is unpredictable. Large fistulas may be easier to repair once the tract is allowed to scar and contract. Conversely, delay of closure may have a negative impact on the quality of life of the patients.
Recurrence of fistula after repair
Multivariate analysis of women who underwent VVF repair demonstrated that recurrence was associated with multiple fistulas, size, etiology of the fistula and presence of urinary tract infection before the repair. Risk of recurrence was fivefold higher for size greater than 10 mm, 4.5 times higher for multiple fistulas and threefold higher for those related to obstetric causes. Interposition grafts were protective.94
POSTOPERATIVE CARE
Considerable debate exists regarding postoperative bladder drainage: how long to drain and whether to use a transurethral or a suprapubic route of drainage. Again, studies are influenced by geography, culture, complexity of the fistula, and technique used. Elkins and coworkers, in a series of 36 repairs, used transurethral drainage for 10–14 days with an overall success rate of 70%.45 These were large obstetric fistulas repaired in a Third World country in a patient population with questionable nutrition. Tancer, in his series of 110 small posthysterectomy fistulas, repaired with the Latzko technique, removed the Foley catheter on the 1st or 2nd postoperative day.13 He had a 100% cure rate. Theoretically, because there are no sutures in the bladder wall with a Latzko repair, the bladder wall can fill without tension, obviating the need for prolonged catheterization. The surgeon should tailor the length of time to drain and route of drainage to the individual patient.
Symmonds recommends a suprapubic catheter for fistulas that involve the urethra, bladder neck, or lower portion of the trigone and for complex fistulas.38 A Foley catheter should not be used. It will produce tension, traction, or irritation on the suture line. Suprapubic catheters also facilitate voiding trials.
Most authors recommend good hydration to ensure irrigation of the bladder and prevent of clots, which could obstruct the catheter and lead to unwanted bladder distention and disruption of suture lines.
CONCLUSION
VVFs and urethrovaginal fistulas represent particularly troublesome complications of obstetric and gynecologic surgery. Despite preventive measures and good surgical technique, these injuries can still occur. Whereas obstetric injury is common in developing nations, fistulas from gynecologic surgery are more common in industrialized countries because of improved obstetric care. Appropriate management regarding timing of repair and surgical approach remain controversial.
REFERENCES
Sims JM: On the treatment of vesicovaginal fistulas. Am J Med Sci 23:59, 1852 |
|
Singhal S, Nanda S, Singhal S. Sexual intercourse: an unusual cause of vesicovaginal fistula. Int Urogynecol J Pelvic Floor Dysfunct 2007;18(5):587-8 |
|
Lee RA, Symmonds RE, Williams TJ: Current status of genitourinary fistula. Obstet Gynecol 72:313, 1988 |
|
Goodwin WE, Scardino PT: Vesicovaginal and ureterovaginal fistulas: A summary of 25 years experience. J Urol 123:370, 1980 |
|
Kelly J: Vesicovaginal and rectovaginal fistulae. J Roy Soc Med 85:257, 1992 |
|
Waaldijk K: Surgical classification of obstetric fistula. Int J Gynaecol Obstet 49(2):161, 1995 |
|
Ojanuga D, Ekwempu CC: An investigation of sociomedical risk factors associated with vaginal fistula in northern Nigeria. Women Health 28:103, 1999 |
|
Wittman AB, Wall LL. The evolutionary origins of obstructed labor: bipedalism, encephalization, and the human obstetric dilemma. Obstet Gynecol Surv 2007;62(11):739-48 |
|
Wein AJ, Malloy TR, Carpiniello VL, et al: Repair of vesicovaginal and ureterovaginal fistulas by a suprapubic transvesical approach. Surg Gynecol Obstet 150:57, 1980 |
|
Bai SW, Huh EH, Jung da J, et al: Urinary tract injuries during pelvic surgery: incidence rates and predisposing factors. Int Urogynecol J Pelvic Floor Dysfunct 2006;17(4):360-4 |
|
Sokol AI, Paraiso MF, Cogan SL, et al: Prevention of vesicovaginal fistulas after laparoscopic hysterectomy with electrosurgical cystotomy in female mongrel dogs. Am J Obstet Gynecol 2004;190(3):628-33 |
|
Meeks GR, Sams JO, Field K, et al: Formation of vesicovaginal fistula: The role of suture placement into the bladder during closure of the vaginal cuff after transabdominal hysterectomy. Am J Obstet Gynecol 177:1298, 1997 |
|
Tancer ML: Observations on prevention and management of vesicovaginal fistula after total hysterectomy. Surg Gynecol & Obstet 175:501, 1992 |
|
Wein AJ: Vesicovaginal fistula. In Resnick MI, Kursh E (eds): Current Therapy in Genitourinary Surgery. Philadelphia, BC Decker, 1987: 209, 213 |
|
Siddiqui NY, Paraiso MF. Vesicovaginal fistula due to an unreported foreign body in an adolescent. J Pediatr Adolesc Gynecol 2007;20(4):253-5 |
|
Hanai T, Miyatake R, Kato Y, et al: Vesicovaginal fistual due to a vaginal foreign body: A case report. Acta Urol Jpn 46:141, 2000 |
|
Arias BE, Ridgeway B, Barber MD. Complications of neglected vaginal pessaries: case presentation and literature review. Int Urogynecol J Pelvic Floor Dysfunct 2008 Feb 27. [Epub ahead of print] |
|
Ramaiah KS, Kumar S: Vesicovaginal fistula following masturbation managed conservatively. Aust N Z J Obstet Gynaecol 38:75, 1998 |
|
Singhal S, Nanda S, Singhal S. Sexual intercourse: an unusual cause of vesicovaginal fistula. Int Urogynecol J Pelvic Floor Dysfunct 2007;18(5):587-8 |
|
Lefort G, Bouche-Pillon MA, Lefebvre F, et al: Congenital vesicovaginal fistula. Commentary and review of the literature apropos of a case. Chir Pediatr 1990;31(2):96-9 |
|
Starkman JS, Meints L, Scarpero HM, Dmochowski RR. Vesicovaginal fistula following a transobturator midurethral sling procedure. Int Urogynecol J Pelvic Floor Dysfunct 2007;18(1):113-15 |
|
Jasaitis Y, Sergent F, Tanneau Y, Marpeau L. Vesicovaginal fistula after transobturator tape. Prog Urol 2007;17(2):253-5 |
|
Marshall VF, Jeffs JD, Sarafyan WK: Urogenital sinus abnormalities in the female patient. J Urol 122:508, 1979 |
|
Kursh ED, Morse RM, Resnick MI, et al: Prevention of the development of a vesicovaginal fistula. Surg Gynecol Obstet 166:409, 1988 |
|
Patil U, Waterhouse K, Laungani G: Management of 18 difficult vesicovaginal and urethrovaginal fistulas with modified Ingelman-Sundberg and Martius operations. J Urol 123:653, 1980 |
|
Moir JC: Vesicovaginal fistula as seen in Britain. J Obstet Gynaecol Br Commonw 80:598, 1973 |
|
Hanash KA, Al Zahrani H, Mokhtar AA, Aslam M. Retrograde vaginal methylene blue injection for localization of complex urinary fistulas. J Endourol 2003;17(10):941-3 |
|
Kuhlman JE, Fishman EK: CT evaluation of enterovaginal and vesicovaginal fistulas. J Comput Assist Tomogr 14:390, 1990 |
|
Volkmer BG, Kuefer R, Nesslauer T, et al: Colour Doppler ultrasound in vesicovaginal fistulas. Ultrasound Med Biol 26:771, 2000 |
|
Sohail S, Siddiqui KJ. Trans-vaginal sonographic evaluation of vesicovaginal fistula. J Pak Med Assoc 2005;55(7):292-4 |
|
Hilton P: Urodynamic findings in patients with urogenital fistulae. Br J Urol 81:539, 1998 |
|
Green DE, Philips GL: Case Report: Vaginal prosthesis for control of vesicovaginal fistula. Gynecol Oncol 23:119, 1986 |
|
Bazi T. Spontaneous closure of vesicovaginal fistulas after bladder drainage alone: review of the evidence. Int Urogynecol J Pelvic Floor Dysfunct 2007;18(3):329-33 |
|
Aycinena J: Small vesicovaginal fistula. Urology 9:543, 1977 |
|
Collins CG, Collins JH, Harrison BR, et al: Early repair of vesicovaginal fistula. Am J Obstet Gynecol 111:524, 1971 |
|
Schoenrock GJ, Cianci P: Treatment of radiation cystitis with hyperbaric oxygen. Urology 27:271, 1986 |
|
Ralph G, Tamussino K, Litchtenegger W, et al: Urological complications after radical hysterectomy with or without radiotherapy for cervical cancer. Arch Gynecol Obstet 248:61, 1990 |
|
Symmonds RE: Incontinence: Vesical and urethral fistulas. Clin Obstet Gynecol 27:499, 1984 |
|
Roth TM, Meeks GR, Blythe J, et al: Massive vesicovaginal fistula associated with a vaginal foreign body: A combined abdominal vaginal repair with gracilis muscle interposition flap [a case report]. J Pelvic Med Surg 2003;9(1):19-22 |
|
Iselin CE, Aslan P, Webster GD: Transvaginal repair of vesicovaginal fistulas after hysterectomy by vaginal cuff excision. J Urol 160:728, 1998 |
|
Flynn MK, Peterson AC, Amundsen CL, Webster GD. Functional outcomes of primary and secondary repairs of vesicovaginal fistulae via vaginal cuff scar excision. Int Urogynecol J Pelvic Floor Dysfunct 2004;15(6):394-8 |
|
Raz S: Atlas of Transvaginal Surgery. Philadelphia, WB Saunders, 1992: 141, 165 |
|
Cruikshank SH: Early closure of posthysterectomy vesicovaginal fistulas. S Med J 81:1525, 1988 |
|
Zacharin RF: Obstetric Fistula. New York, Springer-Verlag, 1988 |
|
Elkins TE, Drescher C, Martey JO, et al: Vesicovaginal fistula revisited. Obstet Gynecol 72:307, 1988 |
|
Lawson JB: Tropical Obstetrics and Gynecology. London, Oxford University Press, 1970 |
|
Martius H: Gynecologic Operations and Their Topographic-Anatomic Fundamentals. Chicago, SB Debour, 1939 |
|
Raz S: Female Urology. Philadelphia, WB Saunders, 1983 |
|
Latzko W: Behandlung hochsitzender Blasen und Mastdarmscheiden Fisteln nach Uterus Extirpation mit hohom Scheidenverschluss. Zentralbl Gynakol 38:906, 1914 |
|
Latzko W: Postoperative vesicovaginal fistulas: Genesis and therapy. Am J Surg 58:211, 1942 |
|
Martius H: Die operative Wiederherstellung der vollkommen fehlenden Harnrohre und des Schiessmuskels derselben. Zentralbl Gynakol 52:480, 1928 |
|
Birkhoff JD, Wechsler M, Romas NA: Urinary fistulas: Vaginal repair using a labial fat pad. J Urol 117:595, 1977 |
|
Elkins TE, DeLancey JOL, McGuire EJ: The use of modified Martius graft as an adjunctive technique in vesicovaginal and rectovaginal fistula repair. Obstet Gynecol 75:727, 1990 |
|
Ingelman-Sundberg AGI: Pathogenesis and operative treatment of urinary fistulae in irradiated tissue. In Youssef AF (ed): Gynecological Urology. Springfield, Ill: Charles C Thomas, 1960: 263 |
|
Hamlin RHJ, Nicholson EC: Reconstruction of urethra totally destroyed in labour. BMJ 1:147, 1969 |
|
Lentz SS. Transvaginal repair of the posthysterectomy vesicovaginal fistula using a peritoneal flap: the gold standard. J Reprod Med 2005;50(1):41-4 |
|
Alagol B, Gozen AS, Kaya E, Inci O. The use of human dura mater as an interposition graft in the treatment of vesicovaginal fistula. Int Urol Nephrol 2004;36(1):35-40 |
|
Gray LA: Urethrovaginal fistulas. Am J Obstet Gynecol 101:28, 1968 |
|
Keettel WC, Sehring FG, deProsse CA, et al: Surgical management of urethrovaginal and vesicovaginal fistulas. Am J Obstet Gynecol 131:425, 1978 |
|
Noble CP: The new formation of the female urethra with report of a case. Am J Obstet Gynecol 43:170, 1901 |
|
Moir JC: The Vesico-Vaginal Fistula. London, Baillière, Tindall & Cox, 1961 |
|
Symmonds RE, Hill LM: Loss of urethra: A report on 50 patients. Am J Obstet Gynecol 130:130, 1978 |
|
Leach GE: Urethrovaginal fistula repair with Martius labial fat pad graft. Urol Clin North Am 18:409, 1991 |
|
Fernandes M, Lavengood RW Jr, Ward JN, et al: Reconstruction of lower ureter and urethra, and closure of vesicovaginal fistula and other bladder defects: Various uses of bladder flap. Urology 1:444, 1973 |
|
Hendren WH: Construction of female urethra from vaginal wall and a perineal flap. J Urol 123:657, 1980 |
|
Omo-Dare P: Reconstruction of the urethra for stricture: Description and evaluation of a technique. J Urol 103:69, 1970 |
|
Nesrallah LJ, Srougi M, Gittes RF, et al: The O’Conor technique: The gold standard for supratrigonal vesicovaginal fistula repair. J Urol 161:566, 1999 |
|
O’Conor VJ, Jr: Review of experience with vesicovaginal fistula repair. J Urol 123:367, 1980 |
|
O’Conor VJ, Jr, Sokol JK, Bulkley GJ, et al: Suprapubic closure of vesicovaginal fistula. J Urol 109:51, 1973 |
|
Ostad M, Uzzo RG, Coleman J, et al: Use of a free bladder mucosal graft for simple repair of vesicovaginal fistulae. Urol 52:123, 1998 |
|
Evans DH, Madjar S, Politano VA, et al: Interposition flaps in transabdominal vesicovaginal fistula repairs: Are they really necessary? Urology 57:670, 2001 |
|
Vyas N, Nandi PR, Mahmood M, et al. Bladder mucosal autografts for repair of vesicovaginal fistula. BJOG 2005;112(1):112-14 |
|
Pettersson S, Hedelin H, Jansson I, et al: Fibrin occlusion of a vesicovaginal fistula. Lancet 28:933, 1979 |
|
Morita T, Tokue A: Successful endoscopic closure of radiation induced vesicovaginal fistula with fibrin glue and bovine collagen. J Urol 162:1689, 1999 |
|
Stovsky MD, Ignatoff JM, Blum MD, et al: Use of electrocoagulation in the treatment of vesicovaginal fistulas. J Urol 152:1443, 1994 |
|
Dogra PN, Nabi G: Laser welding of vesicovaginal fistula. Int Urogynecol J Pelvic Floor Dysfunct 12:69, 2001 |
|
Falk H, Orkin L: Nonsurgical closure of vesicovaginal fistulas. J Obstet Gynecol 9:538, 1957 |
|
Cohen BL, Gousse AE. Current techniques for vesicovaginal fistula repair: surgical pearls to optimize cure rate. Curr Urol Rep 2007;8:413-18 |
|
Muto G, D'Urso L, Castelli E, Formiconi A, Bardari F. Cyanoacrylic glue: a minimally invasive nonsurgical first line approach for the treatment of some urinary fistulas. J Urol 2005;174(6):2239-43 |
|
Sharma SK, Perry KT, Turk TM. Endoscopic injection of fibrin glue for the treatment of urinary-tract pathology. J Endourol 2005;19(3):419-23 |
|
McKay HA: Vesicovaginal and vesicocutaneous fistulas: Transurethral suture cystorrhaphy as a new closure technique. J Urol 158:1513, 1997 |
|
McKay HA. Vesicovaginal fistula repair: transurethral suture cystorrhaphy as a minimally invasive alternative. J Endourol 2004;18(5):487-90 |
|
Miklos JR, Sobolewski C, Lucente V: Laparoscopic management of recurrent vesicovaginal fistula. Int Urogynecol J Pelvic Floor Dysfunct 10:16, 1999 |
|
Tiong HY, Shim T, Lee YM, Tan JK. Laparoscopic repair of vesicovaginal fistula. Int Urol Nephrol 2007;39(4):1085-90 |
|
Dalela D, Ranjan P, Sankhwar PL, et al: Supratrigonal VVF repair by modified O'Connor's technique: an experience of 26 cases. Eur Urol 2006;49(3):551-6 |
|
Sotelo R, Mariano MB, García-Segui A, et al: Laparoscopic repair of esicovaginal fistula. J Urol 2005;173(5):1615-18 |
|
Chibber PJ, Shah HN, Jain P. Laparoscopic O'Conor's repair for vesico-vaginal and vesico-uterine fistulae. BJU Int 2005;96(1):183-6 |
|
Angioli R, Penalver M, Muzii L, et al: Guidelines of how to manage vesicovaginal fistula. Crit Rev Oncol Hematol 2003;48(3):295-304 |
|
Dorairajan LN, Khattar N, Kumar S, Pal BC: Latzko repair for vesicovaginal fistula revisited in the era of minimal-access surgery. Int Urol Nephrol 2007; Sep 21 [Epub ahead of print] |
|
Catanzaro F, Pizzoccaro M, Cappellano F, et al: Vaginal repair of vesico-vaginal fistulas: our experience. Arch Ital Urol Androl 2005;77(4):224-5 |
|
Melah GS, El-Nafaty AU, Bukar M. Early versus late closure of vesicovaginal fistulas. Int J Gynaecol Obstet 2006;93(3):252-3 |
|
Collins CG, Collins JH, Harrison BR, et al: Early repair of vesicovaginal fistula. Am J Obstet Gynecol 111:524, 1971 |
|
Persky L, Herman G, Guerrier K: Nondelay in vesicovaginal fistula repair. Urology 13:273, 1979 |
|
Ayed M, El Atat R, Hassine LB, Sfaxi M, Chebil M, Zmerli S: Prognostic factors of recurrence after vesicovaginal fistula repair. Int J Urol 2006;13(4):345-9 |